On-Going Research Projects
Understanding the Mechanism of Cellulose Hydrolysis by Cellulase Enzymes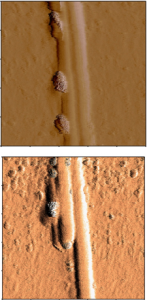
(Graduate student: Akshata Mudinoor and Jennifer Nill)
In this project, we are seeking to understand how to predict productive v. unproductive cellulase interactions with cellulose. One approach focuses on studying the role of cellulose microstructure and surface chemistry on the accessibility of productive binding sites for cellobiohydrolases. We utilize purified Trichoderma reesei Cel7A, a reducing-end specific cellobiohydrolase as a probe to quantify productive-binding. We have found thus far that the organization of cellulose into microfibrils and larger fibrils can limit the accessibility of available reducing ends to T. reesei Cel7A. Fibrillation of the cellulose fibrils appear to be a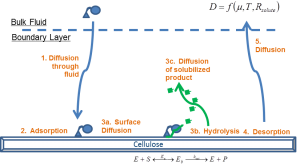 ssociated with improving productive binding leading to higher hydrolysis rates by T. reesei Cel7A. A second approach focuses on studying how we might distinguish between productively bound and non-productively bound T. reesei Cel7A. At this time, we are examining the role of the catalytic domain of T. reesei Cel7A in productive binding to cellulose. Our ultimate goal is to elucidate the mechanisms of cellulose hydrolysis by cellulases, incorporating an understanding of contributions of the cellulose substrate in the mechanisms.
ssociated with improving productive binding leading to higher hydrolysis rates by T. reesei Cel7A. A second approach focuses on studying how we might distinguish between productively bound and non-productively bound T. reesei Cel7A. At this time, we are examining the role of the catalytic domain of T. reesei Cel7A in productive binding to cellulose. Our ultimate goal is to elucidate the mechanisms of cellulose hydrolysis by cellulases, incorporating an understanding of contributions of the cellulose substrate in the mechanisms.
Understanding and predicting how biomass pretreatment affects cellulase accessibility to cellulose in lignocellulosic biomass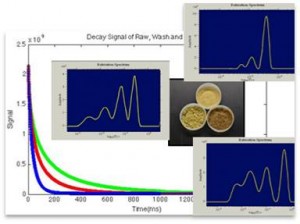
(Graduate student: Nardrapee Karuna)
Bioprocessing of lignocellulosic biomass requires an upstream thermochemical pretreatment preceding enzymatic saccharification to improve the access of cellulose in the biomass cell walls to cellulase enzymes, and to generally render the substrate more enzymatically digestible. Many combinations of temperatures, pressures, chemical catalysts and solvents yield improvements; however, it is not yet possible to predict the impact on downstream sacch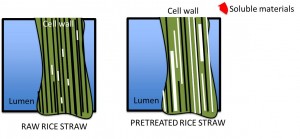 arification rates. We are studying how pretreatment impact productive cellulase engagement to cellulose in pretreated biomass using a combination of analytical tools (1H-NMR relaxometry, MRI and ToF-SIMS) and purified cellulase probes of known substrate specificity.
arification rates. We are studying how pretreatment impact productive cellulase engagement to cellulose in pretreated biomass using a combination of analytical tools (1H-NMR relaxometry, MRI and ToF-SIMS) and purified cellulase probes of known substrate specificity.
Understanding the role of the rheology of saccharification reactions on hydrolysis kinetics
(Graduate student: Maria Cardona)
Increasing solids loadings in saccharification reactions has many advantages that lead to lowered process costs; however, high-solids reactions with lignocellulosic biomass are difficult to mix and are subject to severe mass transfer limitations. We have been active in studying enzyme adsorption and diffusion in high solids conditions, and rheological changes during such reactions. These studies rely on spatial and temporal resolution of the MRI to measure diffusivities of paramagnetically labeled enzymes. Moreover, MRI is used as an inline rheometer to measure changes to the velocity profile  of biomass undergoing saccharification as it is pumped through a pipe. Viscosities are derived from shear stress calculated from the velocity profiles and shear rates calculated from concurrently measured pressure drops. These tools have provided insights into the roles that different cellulases play in reducing reaction viscosities and the role of biomass fiber structure on rheology.
of biomass undergoing saccharification as it is pumped through a pipe. Viscosities are derived from shear stress calculated from the velocity profiles and shear rates calculated from concurrently measured pressure drops. These tools have provided insights into the roles that different cellulases play in reducing reaction viscosities and the role of biomass fiber structure on rheology.
Investigating a new strategy for effective and inexpensive delivery of lipophilic bioactives in foods
(Graduate student: Scott Strobel)
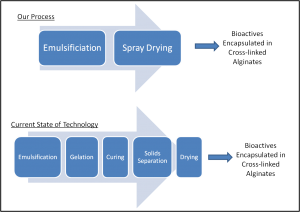 In this project, we address an unmet need for an effective, inexpensive and widely applicable delivery system for lipophilic bioactive compounds in foods. Microencapsulation of hydrophobic bioactives in a protective matrix will allow easier incorporation into foods, preserve activity over the shelf life, mask undesirable flavors and odors, and provide a mechanism for targeted delivery. Microencapsulation in cross-linked alginate is promising for accomplishing these goals. Previously, hydrophobic compounds have been encapsulated in cross-linked alginate microparticles; however, the technology to create these microcapsules requires several time- and energy- intensive unit operations. Our strategy centers on a novel spray drying technology that consolidates the preparation of dry, cross-linked alginate microcapsules into one step. Using this advancement, we are investigating the microencapsulation of lipophilic bioactives in cross-linked alginates with the goals of reducing processing costs, increasing shelf-life and targeting delivery. This four-phase project first involves optimizing the formulation to maximize lipid loadings in the particles and spray-drying yields, examining the stability of the encapsulated lipid phase to oxidation at varying storage times and conditions, characterizing the kinetics of oxygen transport in the alginate particles and determining the release kinetics of the lipophiles in simulated gastrointestinal fluids.
In this project, we address an unmet need for an effective, inexpensive and widely applicable delivery system for lipophilic bioactive compounds in foods. Microencapsulation of hydrophobic bioactives in a protective matrix will allow easier incorporation into foods, preserve activity over the shelf life, mask undesirable flavors and odors, and provide a mechanism for targeted delivery. Microencapsulation in cross-linked alginate is promising for accomplishing these goals. Previously, hydrophobic compounds have been encapsulated in cross-linked alginate microparticles; however, the technology to create these microcapsules requires several time- and energy- intensive unit operations. Our strategy centers on a novel spray drying technology that consolidates the preparation of dry, cross-linked alginate microcapsules into one step. Using this advancement, we are investigating the microencapsulation of lipophilic bioactives in cross-linked alginates with the goals of reducing processing costs, increasing shelf-life and targeting delivery. This four-phase project first involves optimizing the formulation to maximize lipid loadings in the particles and spray-drying yields, examining the stability of the encapsulated lipid phase to oxidation at varying storage times and conditions, characterizing the kinetics of oxygen transport in the alginate particles and determining the release kinetics of the lipophiles in simulated gastrointestinal fluids.





